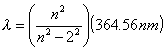
LS-1. Use the Balmer equation below (eq 1) to calculate the wavelengths for the four visible lines in the hydrogen spectrum. What color is each of these lines? What energy transitions are associated with each of these wavelengths?
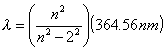
LS-2. Use the generalized formula (eq
2) for energy level transitions to determine the shortest wavelength
in a) the Lyman series where nf = 1; b) the Paschen series where
nf = 3. c) Into what part of the electromagnetic spectrum do these
wavelengths fall?
![]()
LS-3. Startrek-the Fourteenth Generation once featured a race of creatures whose molecular structure was based on silicon. The bond strength between silicon atoms is 2.3 eV. What should be the wavelength of a phasor weapon to cause these creatures to disintegrate?
LS-4. See figure 4 below which depicts the energy-level diagram for the mythical element Mikeyum. a) How much energy does it take to ionize an electron from its ground state? b) A 15 eV photon is absorbed by an atom of Mikeyum. When the atom returns to its ground state, what photon energies will be present? c) What wavelengths are associated with the energies in b) above? Are any of these wavelengths visible? d) What will happen if an 8 eV photon strikes an atom of Mikeyum?
l = 364.56 nm ( n*2 ) / ( n*2 - 2*2) n > 2 equation 1
l = 364.56 nm ( ni*2 ) /(ni*2 - nf*2) ni > nf
Return to Bohr-Rutherford
Model
Last edited 12/26/05