 |
 |
 |
|
| Isaac Newton | Christian Huygens | Thomas Young | |
 |
 |
 |
 |
| Heinrich Hertz | Max Planck | Albert Einstein | Robert A. Millikan |
|
When the solution is simple, God is answering. A. Einstein |
Early in the eighteenth century, Newton published his assertion in a corpuscular theory of light. The experimental data available at the time led him to the conclusion that light consisted of small particles that: a) traveled in straight lines at great speeds; b) reflected from mirrors in a predictable way; and c) changed direction by speeding up when crossing from air to denser materials. While Newton's ideas were challenged by a rival wave theory set forth by Christian Huygens (1629-1695), the triumph of mechanics as laid out in the Principia afforded him the intellectual high ground in this battle of the theories. The wave theory was plausible. but most bet on corpuscles.
The supremacy of a particle theory lasted until 1801 when Thomas Young in England did a series of experiments passing light through narrow slits to show that light had a wave nature. (See Optics, Light as a wave) It became very cumbersome to manipulate the corpuscular theory to explain the pattern of bright and dark bars on a screen when such evidence is prima facie evidence of waves. Young's wave theory of light was therefore solidly ensconced in physics books in the nineteenth century until the discovery of the photoelectric effect in 1887.
 |
 |
 |
|
| Isaac Newton | Christian Huygens | Thomas Young | |
 |
 |
 |
 |
| Heinrich Hertz | Max Planck | Albert Einstein | Robert A. Millikan |
Heinrich Hertz had been working with a cathode ray tube, using
a high voltage power supply to energize the cathode. With this
apparatus he got the same results as other experimenters. But
he also discovered that cathode rays could be produced at a much
lower voltage if the cathode were illuminated with ultraviolet
light. Given that a cathode ray tube current was induced by light
shining on the cathode, this phenomenon became known as the photoelectric effect..
A simple explanation could have been that the UV provides sufficient
energy to help liberate cathode rays and therefore reducing the
high voltage needed to do so. Not so fast. Hertz found that in
order for cathode rays to be produced, the frequency of the incoming
UV had to be above some threshold value. As long as this condition
existed, even a dim UV ray produced a weak cathode ray. But if
the frequency of incoming radiation was below a threshold value,
no cathode rays were forthcoming, not even if the UV beam was
very bright and not even if the exposure time was quite long.
German scientist Max Planck entered the arena in 1900 with a bold concept that light that normally exhibited a wave nature could also have a particle nature as well. An important feature of this scheme is that the two behaviors are not both evident at the same time. He suggested that the energy carried by a wave was delivered in lumps called photons or quanta Furthermore, the energy carried by a photon was directly proportional to the frequency (and therefore inversely promotional to wavelength). Thus, light had a wave-particle duality, a new phrase for the new century. Planck was awarded a Nobel prize for this work in 1918.
Now entering the picture is that soon-to-be rookie of the year, Albert Einstein. From nowhere. Yes, the same Albert who did not speak until he was three; Albert who failed in his first attempt to enter the polytechnic. The same Herr Einstein who graduated with a GPA of 4.6 (on a 6.0 scale). Married with two small children, he worked by day as a patent clerk (third class); at night, he wrote and thought about physics. The year 1905 is called his miracle year (the Latin appears below) during which he published no fewer than five ground-breaking papers. The titles are listed in the box. The papers represent refinement and advancement of work started by others. He, too, stood on the shoulders of giants.
|
On the Electrodynamics of Moving Bodies Does the Inertia of
a Body Depend on Its Energy Content? On a Heuristic Point
of View Concerning the Production and Transformation of Light. On the Movement of Small
Particles Suspended in Stationary Liquids Required by the Molecular-Kinetic
Theory of Heat A New Determination
of Molecular Dimensions |
Einstein suggested that, in order for an electron to be removed from the cathode, a given quantity of energy had to be delivered to the atom. He called this the work function, W. He adopted Planck's quantum theory of light for energy delivery. If an incoming photon cannot sastisfsy the work function, nothing happens. The electron cannot store the energy; two photons cannot gang up on an electron. But if the incoming photon has more energy than the work function, an electron is removed, the excess energy shows up as kinetic energy. As an equation
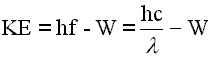 |
It is for this work on the photoelectric effect that Einstein was awarded a Nobel prize in 1921.
Visit these sites for a different view of this topic
http://spiff.rit.edu/classes/phys314/lectures/photoe/photoe.html
http://theory.uwinnipeg.ca/physics/quant/node3.html
Last edited 12/26/05