Nuclear
Fission
"There is no likelihood man can ever tap
the power of the atom."
Robert Millikan, Nobel Prize in Physics, 1923 |
There are two possible
outcomes: If the result confirms the hypothesis,
then you've made a measurement. If the result is contrary
to the hypothesis, then you've made a discovery. |
When Fermi announced in 1934 that his research group
had synthesized new elements beyond number 92, other physicists
began pursuing the quest for new elements. In early 1939 two
German research scientists, Otto Hahn and Fritz Strassmann, bombarded
a chemically pure sample of uranium and noticed after a period
of time the sample contained trace amounts of elements from the
middle of the periodic table. Initially, they were reluctant
to suggest a process for producing these lighter isotopes. That
bold step was taken by Lise Meitner (an aunt to Herr Frisch)
and Otto Hahn who suggested that the atom had been split into
smaller parts in a process they named "fission" borrowing
the term from biology. Portraits of these folks appear below.
 |
 |
 |
 |
|
Otto Frisch |
Fritz Strassman |
Otto Hahn |
Lise Meitner |
Let's do the math.
The fission reaction described immediately
below is one of about 200 possible reactions..Precisely which
fission occurs is determined in part by the speed of the neutron
as well as by where it strikes the target nucleus. let's just
assume that it is representative of most fissions that occur.
A neutron moving at just the right speed strikes an atom of Uranium-235.
the atom splits into several pieces. The pieces are: A) two fragments
from the middle of the periodic table. These are the so-called
nuclear waste materials that no one knows what to do with, (more
about that later) B) the electrons are of no consequence; and
C) there are neutrons (usually to or three) that came away at
high rates of speed. It is the neutrons that make the chain reaction
possible.One neutron produces a fission that produces two neutrons,
which themselves cause four fissions; you get the picture.
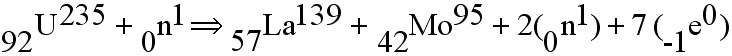
The careful observer will notice that
atomic number (the subscripted numbers tell the number of protons
in each nucleus) balance left and right; so do the mass numbers
(the superscripted numerals which tell the number of protons
and neutrons in each atom.) But now things start to get goofy.
Lets look at the masses of the particles.
|
Consider the masses (in
am )of these particles |
|
U-235 |
neutron |
La139 |
Mo 95 |
2 neutrons |
7 electrons |
|
235.0439 |
1.0087 |
138.8061 |
94.9057 |
2.0174 |
.0038 |
|
mass before |
mass after fission |
|
236.0526 |
235.7330 |
|
|
Lost mass ] |
.3196 am |
|
|
Matter is destroyed, converted to
Energy by E = mc^2 |
|
|
Binding Energy |
4.8 x 10^-11 J |
|
|
|
|
|
The importance of this event in light
of world events to follow cannot be over-estimated. If one adds
the masses of a U-235 atom and the neutron that split it and then
compares that number with the sum of the masses of all of the
fission products produced, a startling conclusion appears. The
masses are NOT the same. Mass has been lost, annihilated, converted
into energy according to the rule predicted by the Einstein equation
E = mc^2. The missing mass is small, typically of the order of
.1% of the mass of the original atom. The Einstein conversion
puts the energy equivalent for this mass at about 300Mev or about
10^-11Joules of energy. If one fission only is to be effected,
this is not much energy. But what if we were to fission a mole
of U-235 having a mass of 235 g (weighing about 1/2 pound and
easily fitting in the palm of your hand). Multiplying 10^-11 Joules
by 10^23 atoms yields 10^12 Joules. This is an enormous amount
of energy. And there is still 99.9% of the original mass left
In order to cause 10^23 fissions to occur,
a chain reaction would probably be necessary. Could such a sequence
be orchestrated? Presumably, the Germans were ready to try. On
August 2, 1939, thirty days before Germany invaded Poland to start
WWII, Albert Einstein, at the behest Leo Szillard, alerted Franklin
Roosevelt of this country's need to pursue a path of research
that would lead to a chain reaction. (See the letter at http://www.dannen.com/ae-fdr.html
Soon thereafter, a joint military-scientific endeavor, code named
the Manhattan Engineering District, was launched. Lt. Gen. Leslie
R. Groves and J. Robert Openheimer, headed the project which may
have been the most comprehensive research project ever conceived
by peoplekind. The chain reaction, a peaceful event, came to pass
on December 2, 1942, in the squash courts at the University of
Chicago. The work there was directed by Enrico Fermi who by now
had moved to the west to escape the Nazi oppression of Jews. In
time, the Manhattan project collected enough fissionable material
for three weapons. The first was detonated in the New Mexico desert
in July 1945. Within the month, the others were delivered to Japan.
Strangely enough, the August 2 letter
represents the only input that Einstein would have on the bomb
project. An avowed pacifist, Einstein spent his last years trying
to bring nations closer together.
 |
 |
 |
 |
|
Leo Szilard |
Enrico Fermi |
J. Robert Oppenheimer |
Leslie R. Groves |
In order to create a fission bomb, one
needs so-called weapons-grade uranium, at least 95% U-235. By
contrast, U235 exists in nature at about 1%; nuclear power plants
need slightly-enriched uranium at 3-5%. There needs to be critical
mass of uranium, enough atoms present to ensure that once the
chain reaction begins, there will be sufficient fuel to cause
it to continue. Additionally, there needs to be critical array
of the fissionable material to ensure that as neutrons are produced,
they will readily find other atoms to fission.
For more information about fission in
general, visit these web sites:
http://howthingswork.virginia.edu/nuclear_weapons.html
Nuclear fission has more peaceful uses
today. Nuclear reactors now power most of the larger ships and
submarines in the US navy. Power reactors still supply a meaningful
fraction of domestic electricity, although no new reactors have
been started since the Three Mile Island accident in Pennsylvania
in 1978. For a case history of a nuclear project gone wrong, use
your favorite search engine to find Chernobyl.
These sites will give you an idea of
how reactors work.
http://howthingswork.virginia.edu/nuclear_reactors.html
http://www.nrc.gov/reading-rm/basic-ref/students/animated-pwr.html
Additionally, there were some applications
of nuclear power that never got very far. Checkout "Operation
Plowshare" at
http://www.geocities.com/RainForest/Andes/6180/history.html#Plowshare
and"Atoms for Peace" on the
Internet.
Return
to Modern V
last edited 12/29/05







