Radiation
Detectors
While Becquerel is given credit for
the discovery of radioactivity, the isotopes responsible for this
event had been emitting radiation forever. Radioisotopes look
like their stable siblings; the only difference between them is
that, besides atomic mass, radioisotopes give off ionizing radiation.It
is this feature we wish to exploit in detection.
In the table that follows,
we compare the ability to ionize matter with the ability to penetrate
matter
|
|
Ionization
capability |
penetrability |
|
alpha
particles |
High |
Low |
|
beta particles |
Medium |
Medium |
|
gamma
rays |
Low |
High |
Alpha radiation turns
out to be a doubly-ionized helium nucleus with a charge of +2.
This property makes it easy to be a good ionizer , stealing electrons
from matter and leaving a trail of ionized flotsam and jetsom
in its wake. Alphas are poor penetrators because their +2 charge
causes them to be repelled by target nuclei. A few sheets of paper
will stop alpha particles which means that alpha detectors must
have detection chambers thin enough to allow alphas to penetrate.
Gamma radiation penetrates
best ( needs lots of matter to absorb them) but is not a very
good ionizer. This is probably the reason we have life as we know
it on this planet. A good ionizer that is a good penetrator would
have probably squelched any life form in the primordial soup phase
our Earth's development.
Beta particles are in between. A few thicknesses of aluminum foil
will stop most of them.
Detectors
To detect these and
other particles, let us exploit the fact that these particles
ionize matter.
Photographic
Plates
|
Photographis plates are coated with a light-sensitive
emulsion that is easily ionized when light or charged partivles
are incident on them. Plates were first used by Becquerel who
discovered radioactivity with them. Remember that he was looking
for X-rays which were known to fog photographic plates. We opened
this unit with a quote fron Louis Pasteur "Chance favors
the prepared mind. " After Roentgen published an account
of his discovery, a man named Smith in England announced that
he had seen his supply of photographic plates become fogged.
He attributed this calamity to the proximity of the plate storage
area to his cathode ray tube. He had his assistant move the storage
area. |
Cloud
chamber
 |
The cloud chamber was developed over
the first quarter of the twentieth century by Nobel laureate
C.T.R.(Charles Thompson Reese) Wilson. When an air mass containing
water vapor undergoes an abrupt drop in pressure, the temperature
of the air goes down and the vapor condenses. Preferentially,
droplets tend to form on ionized particles. If an ionizing agent
is present, its radiation creates a track of ions upon which
visible droplets are formed.
|
Spark
chamber
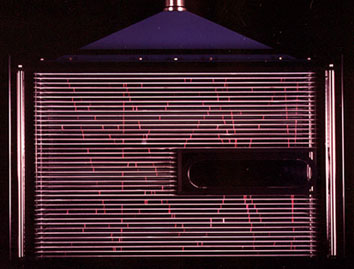 |
At left is an array of thin
metal plates stacked together so that they are electrically
isolated from one-another. A high voltage is placed between adjacant
plates. Gamma rays passing through the array ionize the air molecules
they contact, leaving behind a trail of ions. The high voltage
between the plates causes a small visible arc of light to form. |
Bubble
Chamber
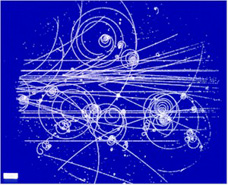
|
The bubble chamber was a very useful
device in the the 1950s through 1980s for witnessing various
sub-atomic interactions. It was conceived by Donald Glaser while
contemplating a glass of beer. Glaser knew that carbon dioxide
comes out of solution to cause bubbles and that this process
occurs most readily in the presence of ions. Glaser used liquid
hydrogen very near its boiling point.If some ionizing event occurs
in this space, the ion trail left behind is made visible by the
bubbles that build on them.
http://mcb.berkeley.edu/site/content/view/103/
|
Dosimeter
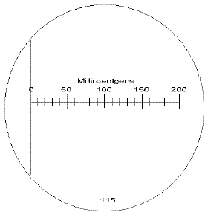 |
A dosimeter is a small unpretentious
device that allows the bearer to monitor cumulative exposure
to X-rays and Gamma rays.The unit, which is the size of a fountain
pen, is given a charge and then worn on a lapel, a collar or
in a pocket protector. A radiation source in the area will ionize
the air creating mobilized ions that will discharge the dosimeter
over the course of time. The unit is read by observing the scale
at one end of apparatus. (See the scale at left.) An employee
checks out a unit upon arrival and returns it to dosimetry at
the end of the day.
Workers in the nuclear industry install
a dosimeter at the beginning of a shift and return it to the
radiation health team whose job it is to monitor levels of exposure
to radiation.
|
Geiger
- Mueller Counter
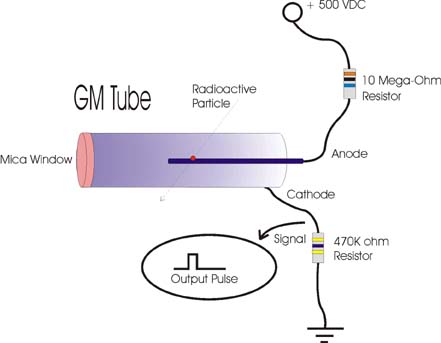 |
A (Hans) Geiger- (Walter) Mueller
tube consists of a closed cylindrical tube filled with an inert
gas and having a wire running along the axis of the tube. A high
voltage is maintained between the wire and the side of the tube.
When a charged particle enters the tube, it ionizes the gas which
creates a place where electrons can flow from cathode to anode.
This current can be made audible as a click from a speaker.
See also http://en.wikipedia.org/wiki/Geiger-M%C3%BCller_tube |
last edited 12/29/05




