|
Becquerel Discovers
Radioactivity |
|
"Chance favors the
prepared mind."
--Louis Pasteur |
 |
In November 1895, Wilhelm Roentgen announced his
chance discovery of a very penetrating radiation which he called
X-rays. The X-rays were produced by high energy cathode rays
colliding with the glass walls of the cathode ray tube. Maxwell
had said that accelerated charges radiate energy; Roentgen's
high energy electrons stopped by the glass walls after all are
accelerating. In his report Roentgen noted that the action of
the cathode rays on the glass caused the emmission of very penetrating
rays he called X-rays. he also noted, almost as a throw-away
line, thet they made the glass fluoresce. This fact prompted
interest in Henri Becquerel, a physicist at the Sorbonne in Paris.
The Becquerel story is a marvelous congruence of the scientific
method and serendipity.
Becquerel was an authority on the phenomena of fluorescence (the
property of a material that causes an immediate emission of light
when bombarded with radiation) and phosphorescence (emission
of light is delayed). Noting the emission of X-rays and the fluorescing
glass tube, he speculated that if there were a connection, other
fluorescent materials should also emit x-rays. In February 1896,
he began a series of investigations of fluorescent materials.
Roentgen had suggested that X-rays could expose photographic
plates, so Becquerel used a plate as his detector. He wrapped
the plate in black paper, put a fluorescent rock on top of the
plate and placed this arrangement on the window sill to expose
it to the sun's UV rays. When he developed the plate it was exposed
and clearly showing the outline of the rock. Looking good. |
 |
 |
 |
|
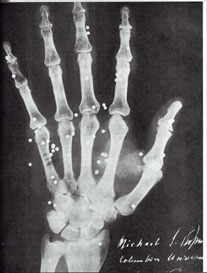
From the top |
|
Among the earliest medical
X-rays. |
|
Becquerel, Roentgen |
|
|
M. Curie |
|
Becquerel was a good scientist. He knew
that a positive result from a single trial was no sound basis
for concluding that the hypothesis--a connection between fluorescence
and the emission of X-rays-- was valid. He tried variations of
the experiment in an attempt to rule out spurious results. For
instance, he placed a glass plate between the flurescsent rock
and the photographic plate. He wondered if the heat of the sun
might boil off a gas from the rock. In the course of these experiments,
he prepared a plate covered with black paper, put the fluorescent
rock on the paper, and put the assembly in a closet for use the
next day. The weather turned dreary for the next three days. Photo
emulsions were not very durable in those days. The plate was of
no use to him, but he decided to develop it anyway. To his dismay,
the plate was exposed, in the outline of the rock and more intense
than ever before. Clearly the sun was not a factor in this turn
of events. At face value, emanating from the rock is a very penetrating
radiation that occurs spontaneously. So what is so special about
the rock? Chemical analysis revealed that the rock contained compound
potassium uranyl-sulfate. Dissecting this compound revealed Uranium
to be the culprit. Uranium was the heaviest of the elements but
its exact place (box 92) on the periodic table was not immediately
determined. Uranium was used in glass-making (today we add lead
to crystal) to give it strength; one hundred years ago, it was
uranium. Uranium was also used in bomb casings; it is both hard
and heavy.
Remember, Becquerel was looking to simulate
a property of x-rays. Instead he got an entirely new effect. Several
new questions came to mind: 1) What was the nature of these rays?
Are they particles? Or are they part of the electromagnetic spectrum?
The same questions were being asked during this time about the
nature of cathode rays. 2) Do other elements emit these strange
rays? Or is this phenomenon confined to just uranium, the heaviest
element? If only one element gave off these rays, then we are
looking at a freak of nature. But if many elements gave off these
rays, then we are looking at a new property of matter. 3) If the
strange radiation is composed of one of several particles that
come from several elements, then what does this phenomenon suggest
about structure deep inside the atom?
The spontaneous emidssion of radiation should make us o all feel
proud as scientists, knowing that we have pushed back that shroud
of ignorance that exists. Trouble is, the trouble is, every time
we answer an old question, we raise two or three new questions.
see also http://www.accessexcellence.org/AE/AEC/CC/radioactivity.html
or http://www.lbl.gov/abc/wallchart/chapters/03/4.html
or http://www.chem.duke.edu/~jds/cruise_chem/nuclear/discovery.html
Radiation Components
Identified
in 1896 for the time being, strange emination
produced by uranium was known as Bequerel rays. These rays were
subsequently identified as three separate kinds of radiation.
Go to http://en.wikipedia.org/wiki/Alpha_particle
Alpha particles were identified by Ernest Rutherford to be doublly
ionized helium nuclei when he did an experiment commonly known
as Rutherford's mousetrap. In this procedure, Rutherford was able
to collect in a sealed glass tube alpha particles emitted by radon.
After a time had expired, he passed a current through the space
occupied by the alphas and obtained light which, when passed through
a diffraction grating, yielded the helium spectrum. It would appear
that some atoms. in their need to reduce instability, jetison
this large fragment. This reduces the atomic number (the number of protons) by two and the mass number (the number of protons PLUS the number of neutrons
) by four. See the box below.
see also
It was J. J. Thompson who identified
the beta particle to have the same charge to mass ratio has the electron,
a coincidence too close to not associate these two particles together.
In some selection process, a neutron is chosen to decay into a
proton which is retained and an electron which is cast off as
a beta particle. In beta decay, the mass number stays the same
while the atomic number increases by one.
go to http://www.triumf.ca/safety/rpt/rpt_2/node23.html
Gamma rays were subsequently determined to be part of the
electromagnetic spectrum and having the shortest wavelength, highest
frequency part of the spectrum. Gamma emission allows a nucleus
to dump energy but does not change mass number or atomic number.
Atoms Are
Changable
Once it was established that atoms
had constituent parts (electrons and something positive to render
the net atomic charge = zero), it was not so far-fetched to wonder
if alphas and betas were the building blocks for atoms. Certainly,
a case could be made that.
Physicists began to study
Uranium, looking to see how it decayed. Today, we know that 99%
of uranium is U-238 and has 92 protons and 146 neutrons. A sample
of U-238 decays into thorium by giving off alpha particles. If
that is the case, then atoms are changable and the 100-plus year
prohibition to atoms changing established by John Dalton must
be discarded. But thorium , Th-234, is also radioactive; it decays
by beta emission into protactinium 234. It turns out that this
element is also radioactive; it, too, is a beta emitter, and decays
into TA-DAH!!! Uranium-234???
The Atoms of
an Element Are Not Identical
Early in the discovery
of radioactivity, some chemists were delighted that vacancies
in the periodic table might be filled by newly discovered elements.
This hope was quickly dashed when it became apparent that there
were likely more new elements than there were vacancies for them.
And while the periodic table could have been replaced, there were
still powerful reasons for retaining it or something close to
it. It was not until 1922 and 1923 that two british scientists,
Frederick Soddy and John Aston won separate Nobel Prizes in chemistry
for their discovery of isotopes ( from the Greek roots Isos meaning
"same" and topos meaning "place". The full
U-238 deccay series is shown in the box below. Rather than being
assembled from end-to-end, this series, and three others like
it, was assembled much like a jigsaw puzzle.
read all about this topic
at
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/radser.html#c3
For an interesting look at life at Cavendish
Laboratory during the Rutherdord years. go to
http://www.phy.cam.ac.uk/cavendish/history/years/rutherford.asp
Click here to go
to derivations
This applet will convey the idea of radioactive
decay
http://www.lon-capa.org/~mmp/applist/decay/decay.htm
This applet shows chart of the nuclides
http://www.lon-capa.org/~mmp/kap30/Nuclear/nuc.htm
Go
to radioactive decay problems
nuclear medicine
http://www.snm.org/index.html#pubawr
And what is the age of the Earth? Go
to
http://scifun.chem.wisc.edu/chemweek/radiation/radiation.html
http://www2.slac.stanford.edu/vvc/theory/halflife.html
Return
to Nucleus within
last edited 12/29/05
uranium institute



